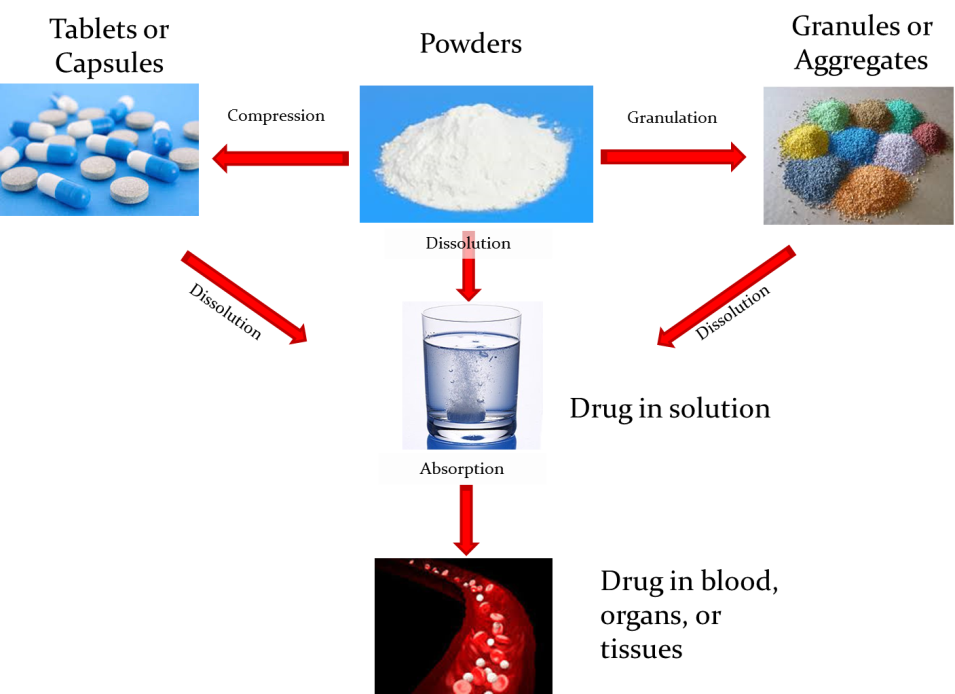
The most widespread diffused administration route is the oral one, and dissolution and absorption are the main phenomena which take place when a drug is ingested. A drug could be administered in several forms, i.e. as tablets, capsules, granules, or aggregates, or even as powders. Independently on the dosage form, the first necessary step for the drug to reach the circulatory system is its dissolution. When the drug enters in contact with the biological fluids, it dissolves, making a solution in the gastrointestinal tract. The absorption is the process by which the drug, passing through the intestinal wall, is transferred in the circulatory system. From the circulatory system it could be distributed in the organs and tissues.
The in vitro models have been developed to reproduce these phenomena. To optimize the pharmaceutical formulations performances, dissolution tests are the most appropriate to study what happens after an oral administration. Indeed, the processes of disintegration, disaggregation, and dissolution studied by the dissolution tests depend on the composition and on the preparation method of the dosage form. These phenomena control the time necessary to the drug to reach the systemic circulation. Thus, the in vitro dissolution tests could be useful to gain information necessary to produce more efficient dosage forms and they are used in the pharmaceutical industry to characterize and develop the dissolution properties of the dosage forms.