One of the most important cause of iron chlorosis is the soil ph, in fact the plant adsorbs the Fe3+ present in the soil by the roots where a reduction from Fe3+ to Fe2+ occur, thanks to the enzymatic reaction. The calcareous soils, for example, due to the carbonates presence, induce a series of reactions which decrease the iron availability, as well as clay soils, limiting the roots development, decrease the possibility to adsorb the iron. To solve the progressive yellowing of plants, due to the chlorophyll content decrease, caused by the iron chlorosis many therapies have been applied as the application of foliar sprays and the dosage of iron salt but both methods have resulted ineffective because, the first one permits only surfaces treatment while in the second one the iron is rapidly converted in insoluble form.
The use of synthetic chelating agents have demonstrated the effectiveness to nurse the iron chlorosis, such as to be nowadays one of the most used substances in the agricultural field.
What is a chelating agent?
The chelating agents are organic macromolecules which thanks to several functional groups are able to establish more than one chemical bond with the metal ion, stabilizing it and increasing its solubility.
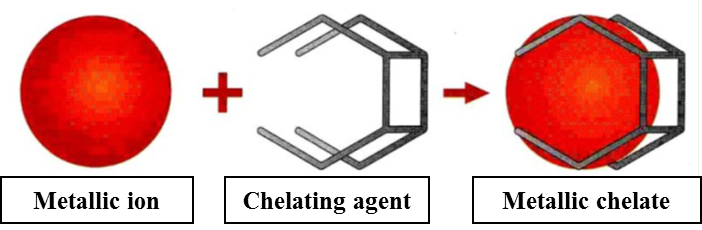
According to the agricultural practice the European commission, by the Regulation (EC) No. 2003/2003, regulates iron and other micronutrient chelates used as they are or incorporated in mixed fertilizers. Six chelating agents, all polyamine carboxylic acids, are permitted for this purpose: EDTA, DTPA, HEDTA, EDDHA, EDDH4MA and EDDCHA. The most efficacious in the therapy of iron chlorosis is the EDDHA chelated iron, characterized by three positional isomeric form which are different between them for the number of bonds with which the chelating agents is bound with the ferric ion. The number of bonds indicates the stability level of complex and for this reason the o,o-EDDHA/Fe3+, where 6 bonds are established between the metallic ion and the EDDHA, is the more incisive because the prolonged release of ferric ions, necessary to the life of plant, is ensured.
Nevertheless, the commercial products contain a mixture of o,o-EDDHA/Fe3+ e o,p-EDDHA/Fe3+ because the production process does not permit the complete chelation of EDDHA in ortho,ortho and moreover the presence of ortho,para ferric EDDHA, where 5 fonds are established between ion and chelating agent, permits an immediate release of ferric ion and then the therapy in the short term.
Mechanism of action of chelating agent
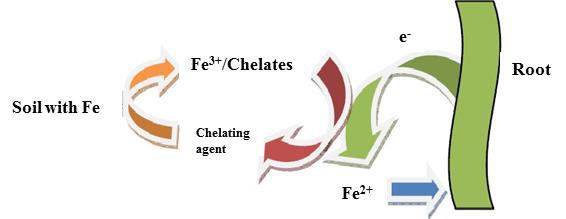
In the iron chelates the chelating agent acts as carrier of iron ion (Fe3+), carrying the ion up to the interface with the roots where it is released and subject to a reduction to Fe2+ after which it is absorbed by roots. The chelating agent, free of ion, returns in the solid phase of soil ready to trap the iron ions present in soil to carry to roots.
